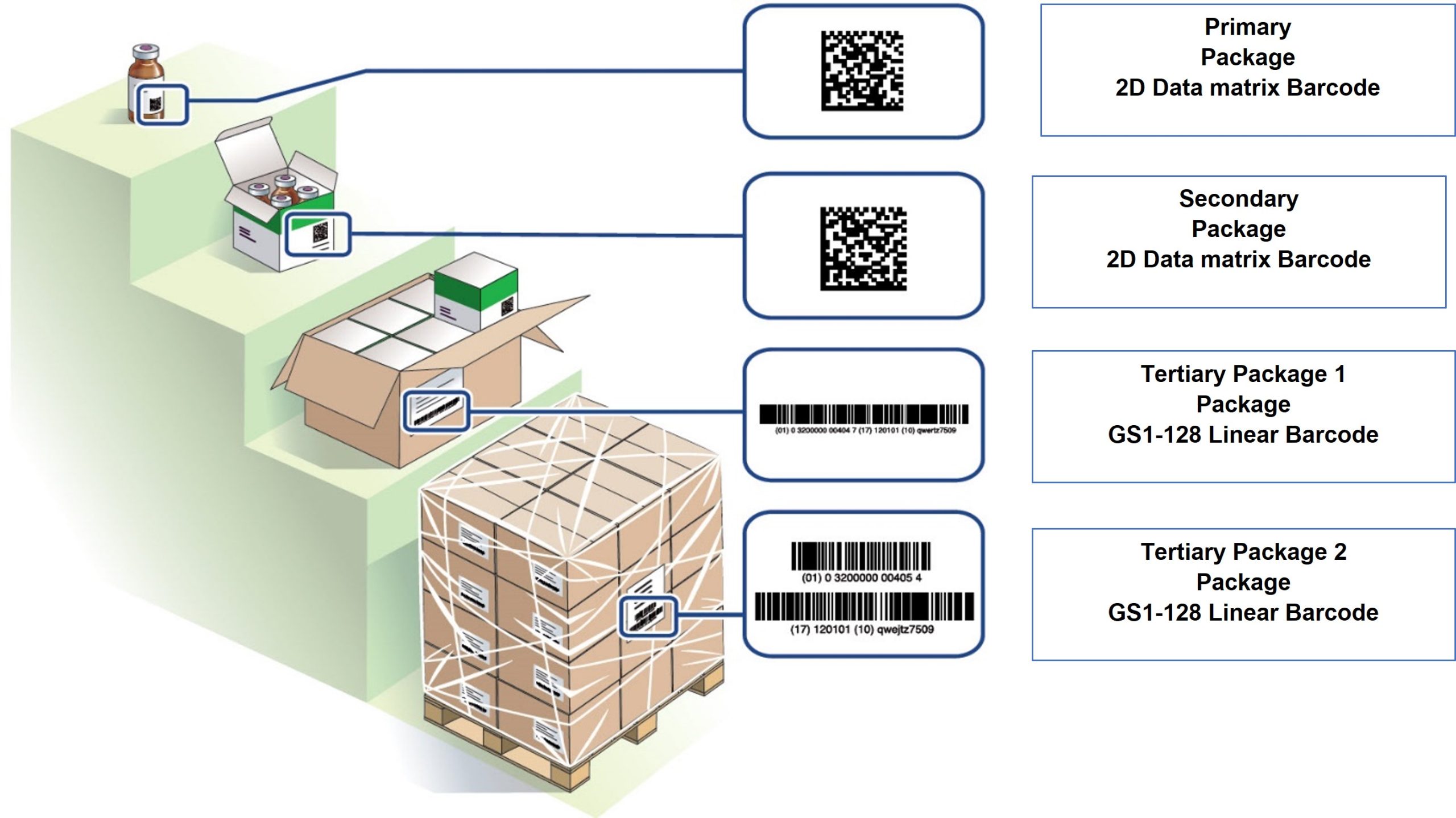
A pilot study was conducted for the traceability of COVID-19 vaccines in Nigeria. The aim was to demonstrate the feasibility of scanning vaccines to provide visibility throughout the supply chain. However, the manufacturers did not provide event data for the products, so the scanning and commissioning of every secondary pack was required. The implementation of the study was limited due to the unavailability of Android-enabled scanners, so personal mobile phones were used instead.
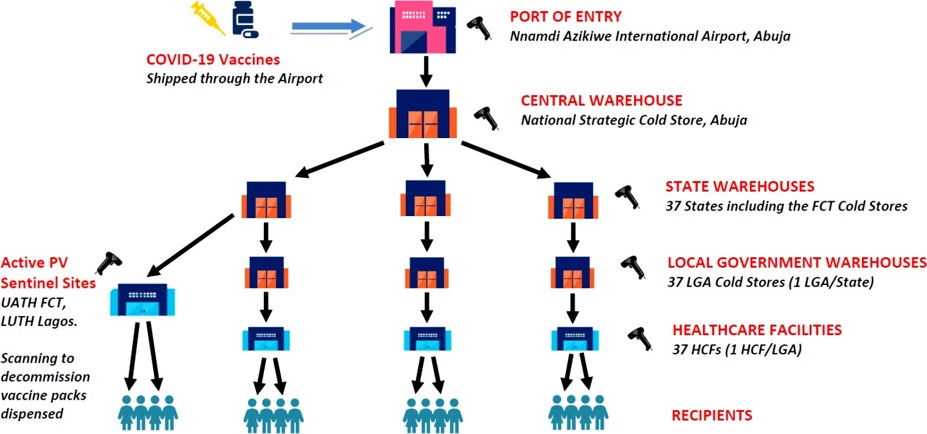
Analysis of the data collected during the pilot showed valid scans from 34 states, while Kogi and Oyo states did not participate and all the scans from Bayelsa State were poorly captured. The batch distribution detected by scanning was largely as expected, but discrepancies were observed in some states and the FCT. The study concludes that automated data capture by scanning has great potential in limiting errors and should be adopted to strengthen the supply chain in Nigeria. The Trackgenic® mobile app used in the study will be further upgraded to ensure validation of GLN and scan data before transmission.
To read more, please click here