Our vision in healthcare
Some of the benefits of adopting GS1 standards in healthcare are:
- Real-Time Supply Chain Visibility.
- Enhancing business processes (e.g inventory management, optimized supply chain efficiency, eProcurement) and lowering costs
- Discouragement of influx of falsified and counterfeit products.
- Discouragement of Diversion of products from the legitimate supply chain.
- Facilitating effective product recalls.
- Tracking pharmaceutical products/medical devices.
- Real time data for Market analytics.
- Brand Protection.
- Simplified Procurement Process.
- Reducing medication errors.
- Linking critical product data to patient records.
- Supporting regulatory compliance.
- Optimizing order, invoice, sales reporting, and chargeback/rebate processes.
- Cost Savings, time savings and better Return On Investment.
GS1 Nigeria works with both the public and private sectors to achieve the objectives above and drive the vision of GS1 healthcare.

Pharmaceutical Traceability Regulation
GS1 Nigeria is pleased to support the implementation of the NAFDAC Traceability Regulation, now officially gazetted. This landmark regulation underscores the commitment to ensuring the safety, quality, and authenticity of medical products throughout Nigeria’s supply chain.
The regulation mandates stakeholders in the healthcare and pharmaceutical industries to adopt traceability practices that enhance supply chain transparency and combat counterfeiting. By leveraging GS1 global standards, such as barcoding and serialization, organizations can seamlessly comply with the regulation and ensure the safety of patients and consumers.

GS1 Healthcare Reference Book
For 10+ years, GS1 Healthcare Global Reference Books have showcased GS1 Healthcare’s many success stories and best practices’ from around the world. Examples of GS1 standards implementation across hospitals, retail pharmacies and the healthcare industry are plentiful. Stay informed about the transformative impact of GS1 in healthcare by exploring our latest case studies and by unlocking key insights and knowledge.
Pharmaceutical Master Data Information
GS1 Nigeria is committed to supporting regulatory compliance and improving data accuracy across the healthcare and pharmaceutical supply chain. The NAFDAC Master Data List serves as a vital resource, providing a centralized database of registered medical products in Nigeria.
By accessing this list, stakeholders can verify product registration details, enhance supply chain transparency, and ensure alignment with regulatory requirements. The integration of GS1 standards further supports accurate product identification and efficient data sharing.

GS1 standards implementation stories in healthcare
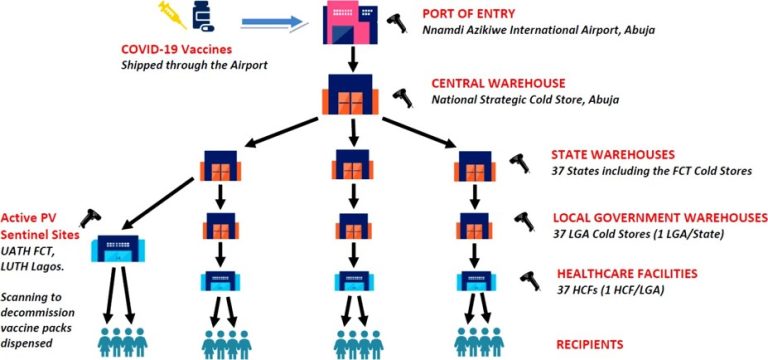
PUBLIC SECTOR PILOT REPORT ON COVID -19 VACCINES: the deployment of GS1 standards in action

Fresenius Kabi is a global leader in the manufacture of generic sterile injectable medicines along with medical technologies for transfusion, infusion, and nutrition.
